Details of the Drug
General Information of Drug (ID: DMTF7JA)
| Drug Name |
(E)-10-nitrooctadec-9-enoic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
10-nitrooleic acid; 10-nitro-9E-octadecenoic acid; 10-Nitro Oleic Acid; (E)-10-nitrooctadec-9-enoic acid; 10-nitroelaidic acid; 875685-46-4; UNII-1N19AGY57Y; CHEMBL561371; 1N19AGY57Y; CHEBI:86285; (9E)-10-nitrooctadecenoic acid; 10-Nitrooleate; 10-nitro-oleic acid; 10-Nitroelaidate; 88127-53-1; 9-Octadecenoic acid, 10-nitro-, (9E)-; E-10-nitrooleic acid; Oa-NO2; 10-nitro-9E-Octadecenoate; (9E)-10-Nitrooctadecenoate; 10-Nitro-9-octadecenoic acid; SCHEMBL1018141; CXA-10; (e)-10-Nitrooctadec-9-enoate; WRADPCFZZWXOTI-BMRADRMJSA-N
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
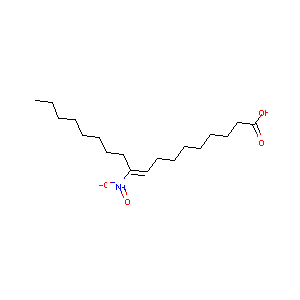 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 327.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 15 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
